
On the efficacy of influenza and COVID-19 vaccines
KARSTEN MONTAG, 14. Juni 2021, 0 Kommentare, PDFNote: This article ist also available in German.
Design and interpretation of the results of efficacy studies
In order to prove the effectiveness of vaccines, randomized controlled trials (RCT) are regarded as the gold standard of evidence-based medical research. The participants in such a study are selected at random and, likewise randomly, assigned to two groups of roughly equal size. The participants in one group receive the vaccine to be tested, while the participants in the other group, also called the control group (hence “controlled” study), receive a placebo or, for example, another vaccine. Ideally, the study is double-blind, which means that both the participants as well as the treating physicians and nurses do not know whether they are receiving respectively giving the vaccine or the placebo.
The special nature of this study design minimizes possible biases, which can occur when using case-control studies, in which, for example, a group of sick people (cases) is compared with a "suitable" group of not sick people (control group). The result of a case-control study essentially depends on the selection of the suitable control group. Studies of this design financed by the pharmaceutical industry are therefore often suspected of selecting the control group in such a way that the vaccine to be tested turns out to be as effective as possible in the end.
But even randomized controlled trials are not free from biasing factors. The British network organization Cochrane Collaboration, or Cochrane for short, founded in 1993 with 79,000 members and supporters from over 130 countries has set itself the goal of promoting evidence-based decision-making on health issues by evaluating and comparing effectiveness studies on vaccines. Using a method called GRADE (Grading of Recommendations, Assessment, Development and Evaluation), the participating scientists evaluate the quality and evidence of studies by other researchers. A study is assigned a lower rating, if, for example, not all of the data that led to the result is published or other criteria such as the random selection of participants cannot be comprehended.
Eventually, the presentation of the results of a study can also have a very biasing effect on laymen. The relative risk reduction of a vaccine of, for example, 95 percent, which is also referred to as its effectiveness, tells nothing at all about how high the absolute risk reduction is and how many people have to be vaccinated for this pharmaceutical measure to have any effect at all. In order to explain how the result of a randomized controlled study is determined, the analysis of a study on a vaccine against tetanus is presented below.
Evaluation of randomized controlled studies using the example of a tetanus vaccination
The first vaccine against tetanus was developed and administered as early as 1890. Clinical studies of today's design were not carried out at that time. The effectiveness assessment is mainly based on a comparison of tetanus infections among American and British soldiers in the First and Second World War. The vaccinated soldiers in World War II contracted far less a tetanus infection than their unvaccinated comrades two decades earlier. Based on these and similar observations, the effectiveness of the tetanus vaccination is no longer questioned and investigated. However, a study on women of childbearing age was carried out in Colombia in 1966 to investigate the effects of the tetanus vaccination on their newborns.
In the double-blind study, the participating women were vaccinated against either tetanus or influenza. After their newborns had seen the light of day, the researchers registered how many children developed tetanus or died from it shortly after birth. Based on this data, the absolute and relative risk reduction is determined as follows.
In the group of mothers who had received the tetanus vaccine at least twice, nine out of 565 newborns developed tetanus, while in the control group, where the mothers had received the flu shot, 49 out of 617 newborns contracted tetanus. The absolute risk of the children developing tetanus is calculated from the number of cases divided by the size of the group. In the tetanus vaccine group the equation 9 / 565 equals 0.016 or 1.6 percent and in the control group 49 / 617 equals 0.079 or 7.9 percent.
The absolute risk reduction is calculated from the difference between the absolute risk of the control group and the absolute risk of the group in which the mothers were vaccinated against tetanus. The absolute risk reduction is therefore 7.9 percent minus 1.6 percent which equals 6.3 percent. The risk of a newborn child developing tetanus is therefore reduced by 6.3 percent if its mother has previously at least twice been vaccinated against the pathogen.
In order to better illustrate the importance of the efficiency of the vaccination, the absolute risk reduction is inverted which results, in this case, in the number of mothers who have to be vaccinated so that one newborn does not get tetanus. This number is referred to as “number needed to vaccinate” or short NNV. 1 / 0.063 results in a number of 16 mothers who have to be at least vaccinated so that one newborn does not get tetanus.
The relative risk is calculated by dividing the absolute risk of the vaccinated group by the absolute risk of the control group. The equation 1.6 percent / 7.9 percent equals to 0.201 or 20.1 percent. The relative risk of newborns developing tetanus if their mothers were vaccinated against the pathogen at least twice is therefore about 20 percent compared to newborns whose mothers were not vaccinated. The relative risk reduction, also called effectiveness, results from the difference to 100 percent, i.e. 79.9 or almost 80 percent.
In a tabular view the figures look like this:
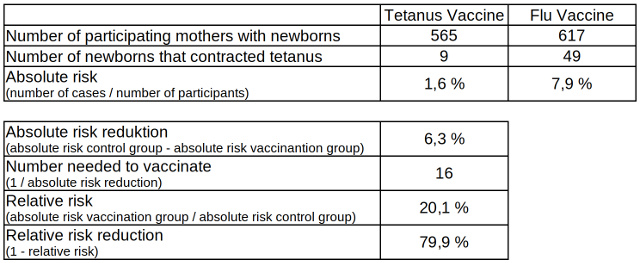
Table 1: Absolute and relative risk reduction of tetanus infected newborns from vaccinated and non-vaccinated mothers, data source: Cochrane Library
For the rest of this article, it is important to understand that the relative risk reduction is only partially related to the absolute risk reduction. If, in the example above, the absolute risk reduction in the vaccinated as well as in the control group had been ten times smaller, i.e. 0.16 percent and 0.79 percent instead of 1.6 and 7.9 percent, the absolute risk reduction would only have been 0.63 percent and the number of mothers to be vaccinated, so that one newborn less would contract tetanus, is almost 160. However, since the quotient of 0.16 and 0.79 also results in 0.201 or 20.1 percent, the relative risk reduction of almost 80 percent would have remained the same.
This means that regarding diseases with a low infection potential, the significance of the absolute risk reduction and the number of people to be vaccinated is considerably higher than that of the relative risk reduction, especially if the disease is largely harmless. In this case, the vaccination risks are to be considered higher in relation to the effectiveness of the vaccination compared to diseases with a high infection potential and predominantly serious consequences.
How serious the consequences of tetanus disease are in newborns without previously vaccinated mothers shows another result of the study in Colombia in the 1960s.
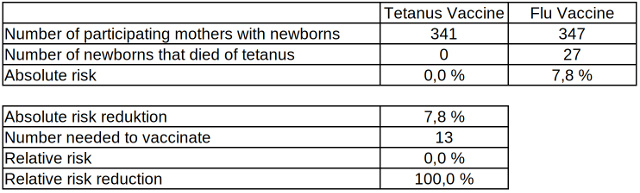
Table 2: Absolute and relative risk reduction of neonatal mortality from tetanus related to vaccinated and non-vaccinated mothers, data source: Cochrane Library
In the group of mothers vaccinated against tetanus, not a single newborn died from the pathogen, while in the control group 27 of 347 fell victim to the disease. An infection and the mortality from tetanus among newborns is significantly reduced if their mothers have previously at least twice been vaccinated against the pathogen.
Since such examinations always represent random samples, clinical studies always include the so-called 95% confidence interval (in short 95% CI). Based on the figures of the sample, it indicates with a confidence of 95 percent a range in which the number of, for example, the relative risk would be after a complete measurement.
Effectiveness of influenza vaccinations
When we talk about having the flu, in most cases this resembles an amateur and often inaccurate self-diagnosis. There are around 200 pathogens that evoke symptoms which are similar to the flu. The share of “real” flu cases, against which a vaccine is available, is just 7 to 15 percent. The official figures for annual flu deaths are also based only on estimates from the statistically determined excess mortality. Only a laboratory test can ultimately confirm whether someone has contracted one of the three main influenza subtypes that have been circulating around the world in recent years. When examining the effectiveness of flu vaccinations, their efficiency and effectiveness against influenza-like diseases are therefore also checked, as there are not always laboratory tests taken to specify the exact origin of the disease.
The first influenza vaccines were developed in the United States in the 1940s. Just a few years later, the researchers realized that the vaccines were losing their effectiveness because, in contrast to the tetanus pathogen, the influenza pathogens mutate steadily and rapidly. Therefore, the vaccines must be adapted to the circulating mutations and administered every year. Due to this fact, the effectiveness of the vaccination not only varies largely, it often can also lead to negative effects. This means that the risk of becoming infected with a mutation not taken into account in the vaccine can increase as a result of the vaccination itself.
The global market volume for vaccinations in 2019 totalled about 33 billion dollars and 90 percent of it is controlled by the four pharmaceutical companies GSK, Pfizer, Merck and Sanofi. The global flu vaccination market was about 4.45 billion dollars in 2019. It is expected to grow up to 7.60 billion dollars by 2027. The large pharmaceutical companies therefore have a considerable economic interest in ensuring that flu vaccinations are administered in large quantities every year, and for this reason they are financing a whole series of studies that are intended to prove the high effectiveness of the vaccines. However, as mentioned at the beginning of this article, the results of such studies are easy to manipulate and independent reviews are required.
Since 1999, the Cochrane research network has been evaluating global studies on the effectiveness of flu vaccinations in adults (16 to 65 years of age) in a constantly updated meta study and is merging the results. The last update from 2016 included 52 randomized controlled studies, which surveyed the effectiveness of the vaccines against influenza and influenza-like illnesses, with a total of over 80,000 participants.
The result of the meta-study reflects a modest light on the efficiency and effectiveness of the flu vaccinations compared to standard vaccinations, for example, against tetanus. The vaccinations reduce the absolute risk of catching the flu from 2.3 to 0.9 percent. The absolute risk reduction is therefore just 1.4 percent, and the number of people to vaccinate so that one influenza case less occurs is 71 (1 / 0.014). The risk of contracting influenza-like pathogens is reduced from 21.5 to 18.1 percent by the vaccination. The absolute risk reduction in this case is 3.4 percent and the number of people to vaccinate to prevent one influenza-like illness is 29 (1 / 0.034).
The absolute risk of being treated in hospital because of an infection with an influenza virus or a pathogen similar to influenza is reduced from 14.7 to 14.1 percent by the vaccine. The absolute risk reduction of 0.6 percent results in a necessary number of 167 vaccinations so that one person less has to be treated in hospital. However, the corresponding studies are classified by Cochrane to have only little evidence and the associated 95% confidence interval of the relative risk reduction, i.e. the effectiveness, ranges from -8 to 15 percent. This means that the actual figure of the effectiveness based on the sample calculations may well be in the negative range. This in turn would mean that the vaccinations could even increase the number of hospitalizations.
By far the most important outcome of the Cochrane review, however, is that a reduction in mortality from influenza or flu-like illnesses due to the vaccinations cannot be proven, neither in adults nor in newborns of mothers who have previously been vaccinated. Since the administration of influenza vaccinations with inactivated pathogens, on the other hand, increases the risk of febrile attacks from 1.5 to 2.3 percent, the question arises how useful this vaccination actually is.
The Robert Koch Institute (RKI) in Germany, the equivalent of the Centres for Disease Control and Prevention (CDC) in the US, has also been reviewing the effectiveness of influenza vaccinations in its annual report on the epidemiology of influenza in Germany since 2001. The last two reports available for the 2017/18 and 2018/19 flu seasons, for the first time, made a distinction between the age groups 0 to 14, 15 to 59 and over 60 years. The largely negative efficacy of vaccinations in the age group over 60 should be cause for concern.
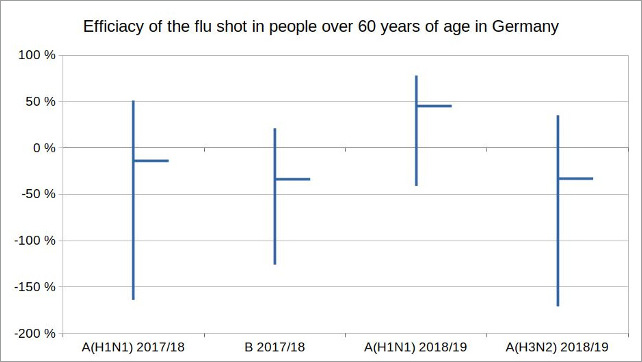
Figure 1: Illustration by the author, data source: Reports on the epidemiology of influenza in Germany 2017 and 2018 by RKI
In the 2017/18 season, of the three influenza subtypes A (H1N1), A (H3N2) and B, mainly only types A (H1N1) and B occurred. The efficacy of the corresponding vaccination in the age group over 60 was -14 percent (95% CI -164 to 51%) for A (H1N1) and -34 percent (95% CI -126 to 21%) for B. In the flu season 2018/19 mainly the subtypes A (H1N1) and A (H3N2) circulated. The efficacy of the vaccination in the age group over 60 was 45 percent (95% CI: -41 to 78%) for A (H1N1) and -33 percent (95% CI: -171 to 35%) for A (H3N2).
So the vaccination has repeatedly increased the potential risk of an infection with influenza, which it was supposed to protect against. It is therefore hardly surprising that in Germany the willingness to be vaccinated against influenza of people over 60 years old has been declining for years, although the World Health Organization (WHO) as well as the German Ministry of Health and the RKI including their leaders Jens Spahn and Lothar Wieler themselves, recommended the flu vaccination, especially for older people.
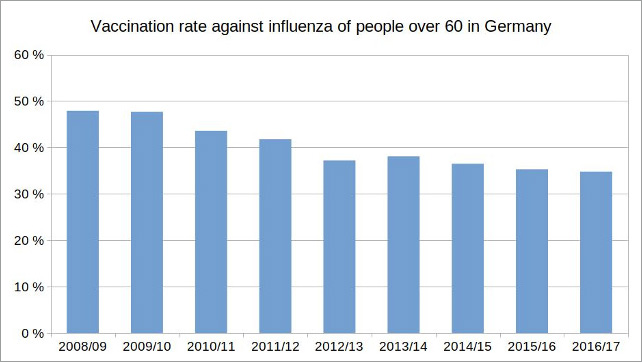
Figure 2: Illustration by the author, data source: Epidemiological Bulletin of January 4, 2018 / No. 1, RKI
Another Cochrane review evaluated the effectiveness of vaccinations of healthcare workers (HCW) in nursing homes related to the mortality of the residents of these facilities from laboratory-confirmed influenza infections. The conclusion of the authors of the review is as follows:
"Our review findings have not identified conclusive evidence of benefit of HCW vaccination programmes on specific outcomes of laboratory-proven influenza, its complications (lower respiratory tract infection, hospitalisation or death due to lower respiratory tract illness), or all cause mortality in people over the age of 60 who live in care institutions. This review did not find information on co-interventions with healthcare worker vaccination: hand-washing, face masks, early detection of laboratory-proven influenza, quarantine, avoiding admissions, antivirals and asking healthcare workers with influenza or influenza-like illness (ILI) not to work [...]. High quality RCTs are required to avoid the risks of bias in methodology and conduct identified by this review and to test further these interventions in combination."
Effectiveness of COVID-19 vaccinations
It is highly expectable that current and future vaccinations against the coronavirus SARS-CoV-2 will have to struggle with similar problems in proving their efficacy as the flu vaccinations, since the pathogen generates symptoms similar to influenza and influenza-like diseases and it probably mutates at the same rate as the influenza virus. The so-called Phase III studies of the currently common COVID-19 vaccines, which have led to emergency approvals by national and international authorities, raise major questions about the efficiency and efficacy of the vaccine.
In Germany, the vaccine against COVID-19 produced by Biontech / Pfizer is mainly administered. The related phase III study, a randomized, double-blinded study controlled by a placebo group and with over 40,000 participants in different countries in the northern and southern hemispheres, must, however, be examined more closely in order to be able to evaluate its informative value. The results of the study funded by Biontech and Pfizer and conducted by Pfizer, which are published in the New England Journal of Medicine on December 10, 2020, show the figures on which the calculation of the efficacy of 95 percent is based.
According to its abstract, the participants, who were all 16 years of age and older, were given two doses of the Biontech vaccine, 21 days apart. Participants were counted as COVID-19 cases,
-
if they contracted a laboratory-confirmed SARS-CoV-2 infection not earlier than seven days after the second vaccination,
-
if the pathogen has never been detected in them before and
-
if they had at least one of the following symptoms: fever, new or increased cough, new or increased shortness of breath, chills, new or increased muscle pain, new loss of taste or smell, sore throat, diarrhea, vomiting.
The absolute and relative risk reductions are as follows:
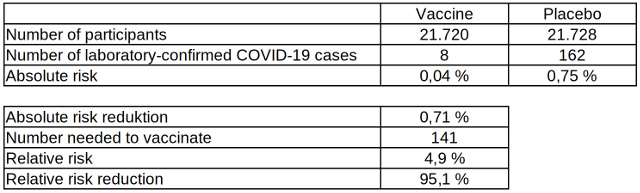
Table 3: Absolute and relative risk reduction of laboratory-confirmed COVID-19 cases after two vaccinations with the COVID-19 vaccine from Biontech, data source: The New England Journal of Medicine
Table 3 shows how low the risk of being tested positive for COVID-19, even without vaccination, actually is compared to the risk of an infection with other pathogens such as tetanus. The absolute risk reduction of 0.71 percent is correspondingly low and the minimum number of people to vaccinate so that one laboratory-confirmed COVID-19 case occurs less is therefore very high. The relative risk reduction of 95 percent may seem high, but almost twice as many people have to be vaccinated compared to influenza vaccinations for one case to occur less.
Since, despite the high number of participants, there was no death due to COVID-19 in either the vaccine or control group, the study cannot make any statement about the reduction of mortality due to the vaccine. According to an evaluation of various Pfizer / Biontech studies as part of the approval process, five people in the placebo group were hospitalized for COVID-19, while none of the participants in the vaccination group were. This results in an effectiveness in reducing hospital admissions of 100 percent, but due to the low number of cases compared to the number of participants the 95% confidence interval ranges from -10 to 100 percent.
However, what the internationally distributed press reports on the efficacy of the Biontech vaccine as well as the assessment of the CDC omit is additional data from the phase III study by Biontech / Pfizer, which was published by the US Food and Drug Administration (FDA). This data, which can be found in a short paragraph on page 42 of the 53-page document, reflects badly on the effectiveness of the vaccination, as, among other things, it also shows the COVID-19-like symptoms without positive confirmation by a laboratory test seven days after the second dose of the vaccine.
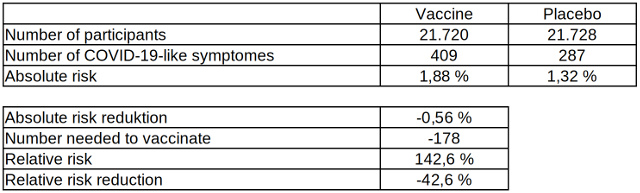
Table 4: Absolute and relative risk reduction of COVID-19-like diseases after two vaccinations with the COVID-19 vaccine from Biontech, data source: US Food and Drug Administration
According to this data, the efficacy of the vaccination for COVID-19-like diseases is -42.6 percent and the absolute risk reduction is -0.56 percent. The risk of suffering a COVID-19-like illness due to a vaccination with the Biontech vaccine is almost as high as its protection against a laboratory-confirmed infection with the pathogen, which, as is well known, can go by with just very few symptoms. As an explanation for this phenomenon, the study's authors speculate:
"It is possible that the imbalance in suspected COVID-19 cases occurring in the 7 days postvaccination represents vaccine reactogenicity [mostly temporary, sometimes violent vaccination reactions – editor's note] with symptoms that overlap with those of COVID-19. Overall though, these data do not raise a concern that protocol-specified reporting of suspected, but unconfirmed COVID-19 cases could have masked clinically significant adverse events that would not have otherwise been detected."
This important information about the level of risk of vaccine side effects in relation to the level of protection of the vaccination against COVID-19 is missing on the websites of the German Federal Ministry of Health and the RKI.
The results of the phase III study of the COVID-19 vaccine from Moderna show a similarly low benefit of the vaccination, despite its high efficacy.
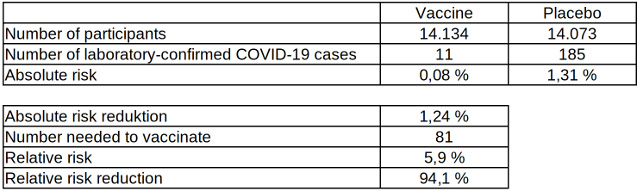
Table 5: Absolute and relative risk reduction of laboratory-confirmed COVID-19 cases after two vaccinations with the COVID-19 vaccine from Moderna, data source: Moderna
Despite the large number of participants, this clinical study as well cannot prove a reduction in mortality due to the vaccine, since no deaths related to the pathogen occurred. Of the 185 COVID-19 cases in the placebo group, three were hospitalized due to COVID-19 symptoms. However, there was also one hospitalization in the vaccinated group due to symptoms similar to COVID-19, which, however, was not confirmed by a laboratory test.
Similar to the Biontech vaccine, the FDA has also published additional data from the phase III study of Moderna about the considerable occurrence of in parts serious vaccine side effects. The high number of these effects in relation to the benefit of the vaccination is also concealed from the pages of the German Federal Ministry of Health and the RKI:
"The most common solicited adverse reactions were injection site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%); 0.2% to 9.7% were reported as severe, with severe solicited adverse reactions being more frequent after dose 2 than after dose 1 and generally less frequent in adults ≥65 years of age as compared to younger participants."
Conclusion
In contrast to vaccines against pathogens that cause diseases with serious and often fatal consequences such as tetanus or diphtheria, the independent medical research network Cochrane estimates the effectiveness of flu vaccinations to be so low that the question arises whether the vaccine’s benefit is adequate to the vaccination risks. The last two studies by the RKI on the effectiveness of influenza vaccination in older people show that the risks of being infected with an influenza virus due to the vaccination can be higher than without it.
With COVID-19 vaccination, as with the flu vaccination, a reduction in mortality could not be proven by randomized controlled trials. Despite the low benefit, both the Federal Ministry of Health and the RKI recommend vaccinations against influenza and SARS-CoV-2.
The vaccines business is worth billions of dollars in the double digits. Due to an aging population in the rich western industrialized countries and the worldwide vaccinations against the novel coronavirus, a future surge in the market volume up to the three-digit billion dollar range is to be expected. All it takes to get there are
-
a respiratory disease that causes a similar number of victims among the elders in relation to the total population worldwide as, for example, the Hong Kong flu at the end of the 1960s – provided that the corona fatalities are counted correctly,
-
influential media that dramatize the disease in such a way that, for example, frightened people go for a walk alone in the forest wearing a surgical mask,
-
unconditional vaccine advocates at the head of health ministries and departments.
Defamation campaigns against experts who repeatedly note the low benefit of mass vaccinations against, compared to other diseases like tetanus or diphtheria, relatively harmless diseases such as influenza and, more recently, COVID-19, did not only start with SARS-CoV-2 infections.
The Wikipedia entry on Tom Jefferson, co-author of the Cochrane review on the efficacy of influenza vaccines, describes him as "controversial" regarding his view on the low benefit of influenza vaccines. In 2018, the expulsion of the Danish medical researcher Peter Gøtzsche, co-founder of the Cochrane network of experts, from the board of the organization caused an éclat in the medical community. He had previously criticized Cochrane reviews, which confirmed the efficacy and safety of vaccines against human papillomavirus, and accused the network of experts of being too close to the pharmaceutical industry. Due to his expulsion from the Board of Directors of Cochrane with a vote of six to five, four other elected leaders left the board in protest.
When the Danish Ministry of Health suspended Gøtzsche from his post as director of the Nordic Cochrane Center at Rigshospitalet in Copenhagen, Denmark's largest hospital, the renowned Stanford professor John Ioannidis wrote an open letter to the Danish Minister of Health. In it he expressed his concern that Gøtzsche's dismissal would severely affect medicine, democracy, freedom of thought and justice and would damage Denmark's reputation as a free country. To date, the suspension has not been revoked.
One wonders if Ioannidis could have already imagined at that time that less than a year and a half later he himself would become the victim of a media defamation campaign due to his critical stance on the measures taken against the COVID-19 pandemic.
(Addendum 23.6.: Because the comparison of absolute risk reductions between COVID-19 vaccines and influenza vaccines caused irritation, it has been removed from the article.)
About the author: Karsten Montag, born in 1968, studied mechanical engineering at the RWTH Aachen, philosophy, history and physics at the University of Cologne, and educational sciences in Hagen. For many years, he was an employee of a management consulting firm with close ties to the trade unions, and most recently department and project manager in a software company that produced and distributed an energy data management and billing system for energy trading.


Diskussion
0 Kommentare